Dawn of Modern Physics¶
| Classical Physics | Modern Physics |
|---|---|
| It is based upon classical mechanics or Newtonian mechanics | It is based upon quantum mechanics |
| It consists of Newton's laws of motion and other laws dealing at macroscopic level. It deals at ordinary everyday life. | It is a discipline of laws of physics that deals at microscopic level as well as macro levels if car is consider as special case. |
Span of classical physics includes:
|
Span of modern physics includes:
|
Ideas of Modern Physics¶
- Light is an electromagnetic wave.
- Light consists of photons, each having energy \(E=hf\).
- Speed of light is a universal constant in vacuum that is independent of motion of source and observers.
- Absorption and emission of electromagnetic waves takes place in the form of packets of energy called photons.
- True laws of physics retain their mathematical form in all inertial frame of reference.
- All the motions and rest are relative.
- There is noting like absolute rest or motion.
- Wave - particle duality.
- Reaction takes some time to replay, after action.
- Matter waves are association with moving particles.
- Einstein Equation's Energy mass relation is \(E=mc^2\)
- Photon can be splitted into a pair of electron and positron, process is called pair production.
- Positron and electron are annihilated to photon.
- Position, momentum, energy and time can't be measured accurately at same time.
Relative Motion¶
- the concept of direction is purely relative.
- The experiments give same results in same frame of reference. In different frame, gives different results.
Frame of Reference¶
- The coordinate system \(x\), \(y\) & \(z\) axes from where observation is made is called frame of reference.
- Inertial frame is that which is either at rest or moving with uniform velocity.
- Non inertial frame is that which is in accelerated motion.
- Earth may be considered as an inertial frame of reference for motion on Earth.
Note
The frame of reference in which the law of inertial is valid is called as inertial frame of reference.
Theory of Relativity¶
- \[ m=\frac{m_o}{\sqrt{1- \frac{v^2}{c ^2}}} \]
- \[ l=l_o\sqrt{1-\frac{v^{2}}{c ^{2}}} \] Lorentz Contraction
- \[ t=\frac{t_o}{\sqrt{1- \frac{v^2}{c ^2}}} \]
- \(E=mc^2\)
- All motion and rest are relative.
- No body moves with velocity greater than velocity of light.
Info
At ordinary speed (as in common life) the results of special theory of relativity are
- \(m=m_o\)
- \(l=l_o\)
- \(t=t_o\)
that is why we don't observe any change in length, mass and time in ordinary life.
NAVSTAR Navigation System¶
- Modern system of navigation called NAVSTAR use results of speed theory of relativity.
- The location and speed anywhere on the earth can be measured accurately up to \(50m\) after \(1hr\) flight and \(20cms^{-1}\) velocity with use of NAVSTAR.
Black Body Radiation¶
Black body is an ideal radiator and ideal absorber.
- The emissive or absorptive power is 1.
- Black body is very bad reflector.
- Black body emits electromagnetic waves.
Intensity Distribution of Black Body¶
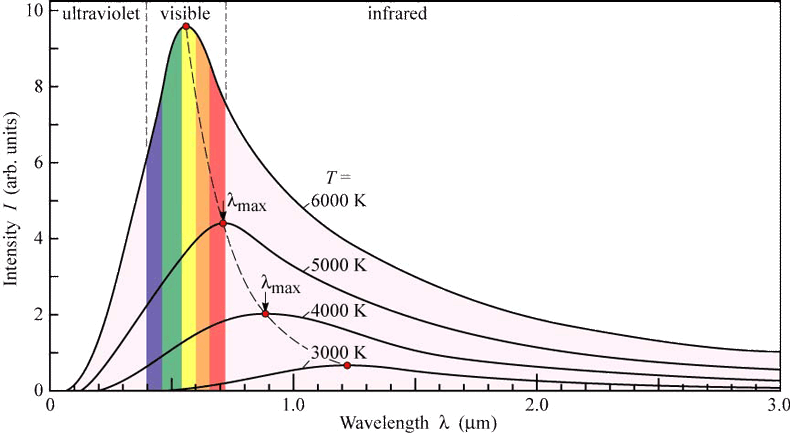
- Lummer and Pringsheim measured the intensity emitted energy of different wavelengths.
- Curve shows the characteristics depending upon temperature.
- At a given temperature, the energy is not uniformly distributed in the radiation spectrum.
- \(\lambda_{max}\times T=\text{constant}=2.9\times10^-3mK\) so, \(T\propto \frac{1}{\lambda}\)
- the area under the curve shows the amount of energy ie emissive power.
- Energy (area of curve) is directly proportional to the fourth power of kelvin temperature i.e. Stephen-Boltzman law is \(E\propto T^4\) $$ E=6t^4 $$ $$ \sigma=5.67\times 10^{-8} Wm^2 K^{-4}=\text{Stephen's constant} $$
- Shape of black body radiation curve is same and independent of material of the body.
-
Classical attempts for explaination of black body radiations are given below:
- Wein's Displacement law holds goof for shorter wavelength.
- Rayleigh Jean's law holds good at longer wavelength.
- Plank's quantum theory explains the black body radiations that absorption and emission of radiation takes place in the form of packets of energy called quanta ie \(E=hf\). It holds good of all wavelengths.
- Max Plank receive noble prize in 1918 in physics for discory of energy quanta.
The Photons¶
- Einstein presented the idea of light energy consisting of packets of electromagnetic energy.
- Max plank explained the emission and absorption by the atoms from a black surface is in the form of invisible packets called quanta.
- Max plank put discontinuous (granular) nature of light.
- The beam of light with wavelength \(\lambda\) consists of stream of photons traveling at speed \(c\) and carrier energy \(hf\).
- As \(E=mc^2\) and \(E=hf\)
then \(mc^2=hf\) and \(E=hf\)
\(mcc=hf\) $$ mc=\frac{hf}{c} $$ As \(mc=p\) and $$ f=\frac{c}{\lambda}p= $$ momentum.
then $$ p=\frac{h}{\lambda} $$ - Emission or absorption of energy is applied to any oscillating system.
Interaction of Electromagnetic Radiation with Matter¶
It is of three types depending upon the energy of photon.
- Photo electric effect \(hf<0.1MeV\)
- Compton effect \(0.1MeV<hf<1MeV\)
- Pair production \(hf>1MeV\)
Photoelectric Effect & Photon Theory of Light¶
- When light is incident on the surface of a metal electrons are emitted from it. This phenomenon is known as "Photoelectric effect."
- The photoelectric effect supports the quantum nature of light.
- Emitted electrons are known as photoelectrons
- This effect is based on the principle of energy conservation.
- Photoelectric effect was first explained by Einstein for which he was awarded Nobel Prize in the year 1921.
Photoelectric Effect¶
- The emission of electrons from metallic surface, when light of specific short wavelength is incident on it is called "Photoelectric effect".
- Hallwach applied some potential difference across two \(Zn\) plates in a quartz vacuum tube and studied the flow of current.
- When ultraviolet light is incident on cathode current flows in the circuit which vanishes when no light falls.
- When light falls 0n anode the current in the circuit is negligible.
Effect of Intensity of Incident Light on Photoelectric Current¶
- When the intensity of light of frequency more than the threshold frequency is increased the number photoelectrons increase i.e., the photoelectric current also increase. Photoelectric current, $$ i \propto I $$ where \(I=\) Intensity of light
Effect of Potential on Photoelectric Current¶
- On increasing the potential current first increases and at a fixed potential reaches a maximum value known as saturation current.
- At a fixed negative potential the value of photoelectric current is zero. This negative potential is called stopping potential.
- The stopping potential is proportional to the maximum kinetic energy of photo electrons.
- The stopping potential depends on the frequency of the incident light.
- The stopping potential does not depend on the intensity of the incident light.
Effect of Frequency of Incident Light on Photoelectric current¶
A simple linear relation exists between stopping potential (maximum energy of emitted electron) and frequency of incident photon.
Laws of Photoelectric Effect:¶
- The rate of emission of photoelectrons from a metallic surface is proportional to the intensity of incident light.
- If the frequency of incident light is less than a specific minimum (whatever the intensity of light) electrons will not be ejected from the surface. This minimum (threshold) frequency is different for different metals. The photo energy to corresponding to threshold frequency is known as work function of metal.
- The maximum K.E. of emitted photoelectrons is proportional (linearly related) to the frequency of incident light but does not depend on the intensity of incident light.
- The time interval of incidence of light on the metallic surface and electron emission is negligible (less than \(10^{-8}s\)), i.e, the process of electron ejection is instantaneous.
Parameters of Photoelectric Effect¶
- Work function \((\phi_o)\): The minimum energy required to eject an electron from the metal surface is known as its work function.\(\quad\phi_o=hf_o\)
- It depends upon:
- The impurities present on the surface of the metal.
- The nature of metal.
- Its unit are \(eV\), \(joule\) and \(erg\).
-
It is a property of material and not of emitted electron.
-
Photo sensitive material: Those elements that eject photons when high frequency light is incident on the material are called photo sensitive material.
- Saturated photoecurrent: The maximum value of photocurrent is called saturated current.
- Due to stopping potential the work done by electrons is equal to the maximum kinetic energy of electrons.
-
Einstein's Explanation of Photoelectric Effect: Light has dual (wave and particle) nature. In interaction of light with matter, light acts as particle. According to quantum theory, the exchange or propagation of light is in the form of small energy packets called photons. $$ E=hf= \frac{hc}{\lambda} $$
-
Einstein's Photoelectric Equation
-
Important Graphs
-
Failure of Classical Theory to Explain Photoelectric Effect The wave theory of light completely failed to explain the experimentally established facts about photoelectric effect.
- The fact that maximum kinetic energy of photoelectrons does not depend on intensity of incident radiation.
- The existence of a threshold frequency or wavelength.
For your Information
A weak beam of radiations having frequency more than threshold frequency, can eject a photo electron while an intense beam of frequency lesser than threshold cannot eject a photo electron.
Photo Cell¶
- A photocell is based on photoelectric effect.
- A simple photocell consists of a glass bulb with a thin anode rod and a cathode of an appropriate metal surface.
- Sodium or potassium cathode surface emits electrons for visible light.
- Cesium coated oxidized silver emits electrons for infrared light.
- Some surface selenium or silicon emits electrons for ultraviolet rays, X-rays and \(\gamma\)-rays.
Applications of Photocells¶
- Security system
- Counting systems
- Automatic door systems
- Automatic street lighting
- Exposure meter for photography
- Sound tracks of movies
- Solar panels in watches and other appliances
Compton Effect¶
When a photon hits with an electron, it scatters with frequency less than that of incident photon; it is known as compton effect.
- Usually X-ray photons are used because of high energy \((\ge 17.5 KeV)\).
- Change (increase) in wavelength is called Compton Shift. $$ \Delta\lambda=\lambda_f -\lambda_i $$ $$ \Delta\lambda=\frac{h}{m_o c}(1-\cos\theta) $$
- $$ \Delta\lambda=\frac{h}{m_o c}=2.43\times 10^{-12}m $$ is called Compton Wavelength.
- \(\Delta\lambda=0\) when \(\theta=0^{o}\).
- $$ \Delta\lambda=\frac{h}{m_o c} $$ when \(\theta=90^{o}\).
- $$ \Delta\lambda=\frac{2h}{m_o c} $$ when \(\theta=180^{o}\).
- Photoelectric effect and Compton effect are strong evidences that e.m waves behave as particle (photon).
- Compton effect proves photon theory of light.
Pair Production¶
Decomposition of photon into electron, positron pair is called pair production.
- Pair production can take place only if photon is greater than \(1.02 MeV\).
- Energy equation for pair production is given as $$ hf = 2m_{o}c^ {2} + K.E_{e^ {-}}+K.E_{e^ {+}} $$
- Rest mass energy of electron or positron is \(m_o c^{2}=0.51 MeV\).
- Condition for pair production is that \(hf\gt 2m_o c^{2}\).
- Pair production can not take place in vacuum.
- The interaction usually takes place in the electric field in the vicinity of a heavy nucleus so that there is a particle to take up recoil energy and momentum is conserved.
- Pair production is materialization of energy.
Annilhilation of Matter¶
Reverse process of pair production is called annihilation of matter.
- It involves conversion of mass into energy.
- Two photons are produced by the annihilation of electron and positron.
- Two photons produced move in opposite direction to obey the law of conservation of momentum.
- Each photon has energy of \(0.51MeV\) equivalent to rest mass energy of electron.
Antimatter¶
- P.A.M Dirac theoretically predicted antimatter in 1928.
- Anderson discovered positron during study of spectrum of cosmic rays in 1932.
- Every antiparticle has same mass, same spin but opposite magnetic moment and charge to its respective particle.
| Particle | Antiparticle |
|---|---|
| Electron | Positron |
| Proton | Antiproton |
| Neutron | Antineutron |
| Neutrino | Antineutrino |
| Earth | Black Hole |
De-Broglie's Hypothesis (Wave particle duality)¶
All the moving particles behave as waves called matter waves or particle waves. The wave length associated with moving particles is given by $$ \lambda=\frac{h}{mv} $$ $$ mV = \text{momentum of particles} $$ $$ \lambda=\frac{h}{p} $$ $$ \lambda\propto\frac{1}{m}\qquad\lambda\propto\frac{1}{v} $$
An object of large mass and ordinary speed has such a small wavelength that its wave effects such as interference and diffraction are negligible.
Davisson and Germer Experiment¶
- Germer and Davisson using low energy electron beam provided experimental confirmation of de-Broglie's hypothesis. They showed that electrons are diffracted from metal crystals in exactly the same manner as X-rays or any other wave.
- The electron beam of energy \(Ve\) is made incident on a nickel crystal. The beam diffracted from crystal surface. The wavelength associated with the moving electrons is given as
$$ \lambda=\frac{h}{mv} $$ $$ mv=\sqrt{2mVe} $$ $$ \lambda=\frac{h}{\sqrt{2mVe}}\quad\text{where V is accelerating potential} $$ $$ V=54v $$ $$ \lambda=1.66\times 10^{-10} m $$
- This beam of electrons diffracted from crystal surface was obtained for a glancing angle of \(65^{o}\). According to Bragg's equation \(2d\sin\theta=m\lambda\).
- For \(1^{st}\) order diffracting \(m=1\).
- For nickel \(d=0.91\times 10^{-10}m\).
- Which gives \(\lambda=1.65\times 10^{-10}m\).
- Prince Louis Victor de Broglie received the 1929 noble prize in physics. Clinton Joseph Davisson and George Paget Thomson shared the Nobel Prize in 1937 for their experimental confirmation of the wave nature of particles.
- Electron microscope is a practical application of wave particles duality.
Heisenburg's Uncertainty Principle¶
It states that following pairs of quantities can't be measured with perfect accuracy at same time
- Linear momentum and position
- Energy and time
Such quantities are called conjugate quantities. Mathematically,
$$ \Delta p=\frac{h}{\lambda} $$ $$ \Delta x=\lambda $$ $$ \Delta p.\Delta x=h $$ $$ \Delta E.\Delta t=h $$
- To increase accuracy in measuring position, we should use short wavelength.
- To increase accuracy in measuring momentum, we should use long wavelength.
Important Considerations¶
- A ball falling freely appears to fall straight to stationary observer and falling along curved path to moving observer. It is due to relative motion.
- If \(V= 0.999C\) then \(m = 22.4 m_o\)
- Millikan determined the values of \('e'\) and \('h'\).
- Human body emits e.m waves in infrared region at only \(310K\).
- If \(V\ge 2.6\times10^{8} m/s\), then \(l=l_o / 2\quad\&\quad m=2m_o\)
| # | Name | Wavelength Range (\(m\)) | Frequency Range (\(Hz\)) | How Produced |
|---|---|---|---|---|
| 1 | Gamma rays | \(6\times10^{-13} - 1\times10^{-10}\) | \(5\times10^{20} - 3\times10^{19}\) | Nuclei of atoms |
| 2 | X-rays | \(1\times10^{-10} - 3\times10^{-8}\) | \(3\times10^{19} - 1\times10^{16}\) | Bombardment of high \(Z\) taregt by electron |
| 3 | Ultraviolet rays | \(3\times10^{-8} - 4\times10^{-7}\) | \(1\times10^{16} - 8\times10^{14}\) | Excitation of atoms and vacuum spark |
| 4 | Visible rays | \(4\times10^{-7} - 8\times10^{-7}\) | \(8\times10^{14} - 4\times10^{14}\) | Excitation of atoms, spark and arc flame |
| 5 | Infrared rays | \(8\times10^{-7} - 3\times10^{-5}\) | \(4\times10^{14} - 3\times10^{13}\) | Excitation of atoms and molecules |
| 6 | Heat radiation | \(1\times10^{-5} - 1\times10^{-1}\) | \(3\times10^{13} - 3\times10^{9}\) | Heating |
| 7 | Microwave | \(1\times10^{-3} - 3\times10^{-1}\) | \(3\times10^{11} - 1\times10^{9}\) | Oscillating currents in special vacuum tubes |
| 8 | Ultra high radio frequencies | \(1\times10^{-1} - 1\) | \(3\times10^{9} - 3\times10^{8}\) | Oscillating circuits |
| 9 | Very high radio frequencies | \(1 - 10\) | \(3\times10^{8} - 3\times10^{7}\) | Oscillating circuits |
| 10 | radio frequencies | \(10 - 10^{4}\) | \(3\times10^{7} - 3\times10^{4}\) | Oscillating circuits |
| 11 | Power frequencies | \(5\times10^{6} - 6\times10^{4}\) | \(60 - 50\) | Weak radiations from AC circuits |